Absolute Stereochemistry
Determining and Differentiating Between R and S Stereocenters
Since it’s presumed the reader has spent some time learning about chirality, we’ll just go straight into absolute stereochemistry. What makes it absolute? The fact that three chemists, Cahn, Ingold, and Prelog who developed them have these rules named after them is no surprise. The “C-I-P” rules are not difficult to understand.
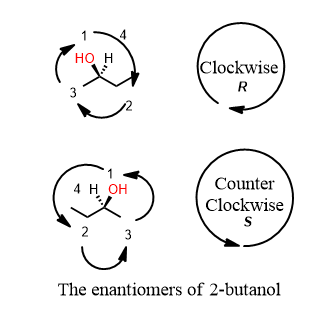
How are we to determine if an atom is a chiral center? Prioritizing the groups is something you must do and also helps you detect a chiral center. In (R)-2-butanol below you can see that there is a carbon with a wedge and dash coming off of it. The first group is OH. O has an atomic number of 8. That beats H (which is the lowest group, 4). What about two and three. There is an apparent tie between the methyl and the ethyl sticking off the chiral center. It is not a tie because of what is attached, in turn, to the atom we went outward to prioritize. The methyl carbon is attached to HHH (H+H+H=3) The ethyl wins because the carbon that is one away is attached to (C+H+H=8). So the groups are 1=OH, 2=ethyl, 3=methyl, 4= hydrogen.
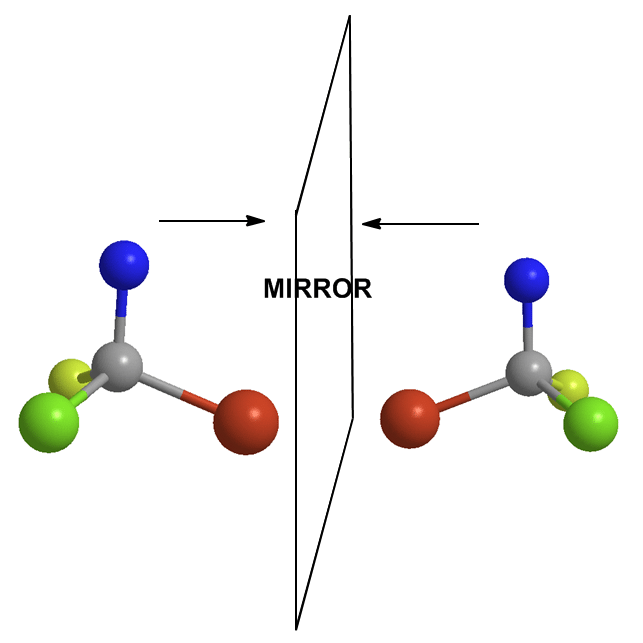
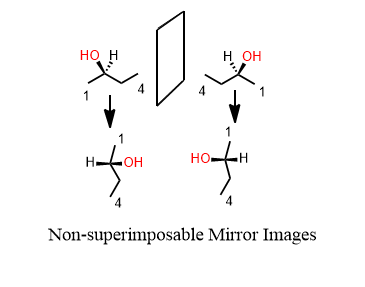 If it has one or more chiral centers, the entire molecule is probably chiral. Yes I said that correctly. A central atom has four different groups on it. Therefore, the molecule has a mirror image that doesn’t overlap perfectly on itself. This mirror-image is said to be the enantiomer of the original. If one is R, the other is S and vice versa.
If it has one or more chiral centers, the entire molecule is probably chiral. Yes I said that correctly. A central atom has four different groups on it. Therefore, the molecule has a mirror image that doesn’t overlap perfectly on itself. This mirror-image is said to be the enantiomer of the original. If one is R, the other is S and vice versa.
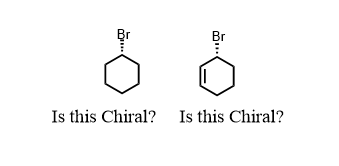
The bromocyclohexane on the left is not chiral. It has no chiral center. There is no way to find four different things on that bromine-containing carbon. The ring has symmetry to it. Chirality is all about asymmetry. The molecule on the right, 2-bromocyclohexene is chiral because the alkene double bond differentiates the two carbons around the chiral center. So:
- Prioritize the group around the chiral center from 1 to 4 based on atomic number.
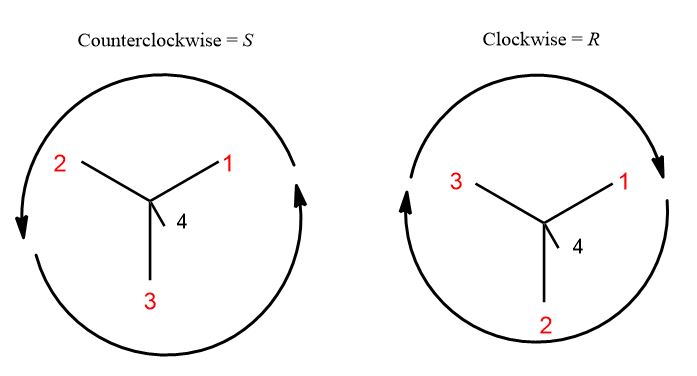
How can we create a way of designating and distinguishing molecules that are non-superimposable mirror images? This is no simple accomplishment. But how it happened was like this. Four things is too many to worry about. So, we would have a model built and in hand, staring at the chiral center holding the lightest (lowest priority) group in one’s hand you’d observe three groups projecting off of it just like a Newman Projection would. They can only go in two different directions: clockwise from 1 to 3 and counterclockwise from 1 to 3. S= left (L. sinister); R = right (L. rectus). The general idea:
Absolute Stereochemistry
Recall that prioritization occurs at the point of first difference. As soon as an atom has a higher atomic number or set of atomic numbers than it takes priority one, then two, three, and finally four. Once you’ve prioritized all the groups, draw a circle from one to two to three, ignoring 4 (you shouldn’t be able to see the lowest priority group, 4 because these rules were invented by imagining oneself holding the lowest priority group in your hand and looking at the remaining three groups.
2. Ask yourself: “Is the lowest priority group pointing away from you (is it a dashed line)? REMEMBER the answer to this question.
Of course, we don’t have the luxury of models on an exam or have access all the time. What you see is this and here is the tricky part. Does the circle of prioritized groups go clockwise or counterclockwise?
3. Draw a circle from 1 to 2 to 3 back to 1, ignoring group 4. If it’s clockwise, it’s R. If it’s counterclockwise, it’s S.
However, remember the answer to step two? Is the lowest priority group pointing away? If the answer is “yes” leave the circle you got alone. If the answer is “no” switch from R to S or from S to R to correct it.
4. If you said “no” to question 2, switch your answer based on the circle you got.
There is more to absolute stereochemistry (a lot more) however this is the minimum ability for undergraduates. Absolute stereochemistry doesn’t go beyond this except for details.