The Organic Chemistry Molecular Model Set and You
It is a good idea and probably a requirement that you purchase (or borrow or use any that happen to be available). Whether you use it a lot or not, a molecular model set for organic chemistry is advisable. As you might expect, a scale model allows one to see how many bonds can form to specific hybridizations of atoms.
What is important is to get perfectly clear that carbon can have bonds arranged in only three specific geometries or hybridization states. These are (109.5o = sp3 / 120o = sp2 / 180o = sp). This also indicates the number of geometries (hybridized atoms have bond angles and VSEPR guidelines apply whether it is single, double, or triple-bonded). Not just carbon, nitrogen, oxygen and the halogens, even boron, all do this.
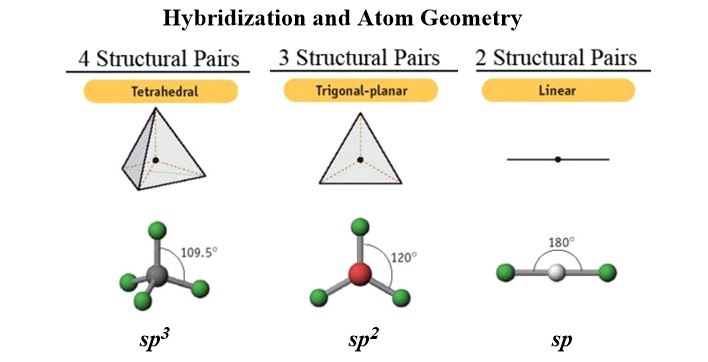
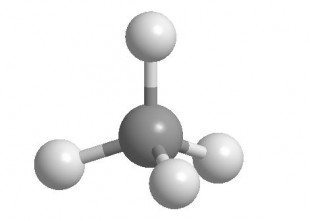
For a single atom, the individual atoms in a model-kit shows you the geometries of the bonds around it (recall, VSEPR Guidelines). The positioning of holes around a model's atoms, tell you their hybridization since the model's atoms have fixed angles depending on where the holes are.
Get clear also on the angles between atoms because drawing (which you are hopefully doing constantly while studying) is easier after you've seen them in a tangible form. The relative lengths of bonds between different elements are differently colored in a good model set. If you build larger and larger molecules you get a rough idea of their time-averaged shape. A model gets you familiar with atomic geometry of 2nd row atoms at the start: tetrahedral, trigonal planar, and linear. Bond angles and geometries are absolutely essential. One advantage of a molecular model set is that it gives you a better idea of how to draw molecules from the beginning.
What Model Sets Are For. How useful this is depends completely on you.
There are several ways to represent organic compounds in two dimensions on paper. Pentane, an alkane, is an example that has five carbons. Systematic naming of compounds (nomenclature) is covered later. All carbons in alkanes are single-bonded to hydrogen (or "saturated" with hydrogens) so that the carbons obey the octet rule. Representing compounds in 2-D, by means other than its name can be done in several ways specified as formulas. A molecular model set can clarify these representations from the beginning.
Lewis structures are made by bookkeeping valence electrons until molecules have all filled orbitals (the reason for the octet rule). If you connect carbon instead of hydrogen on any of them, the chain begins to get larger, changing its composition, name etc.
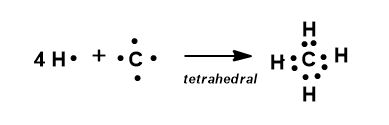
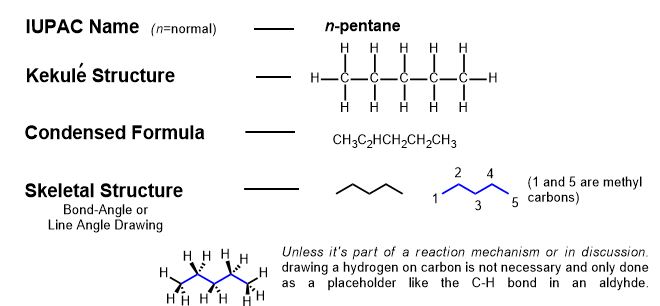
Only the skeletal structure implies geometry correctly, it is the fastest way to draw and write and the most clear representation.
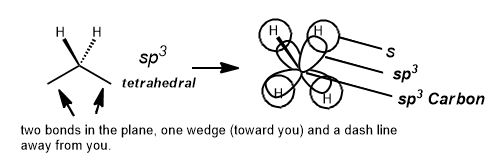
All of these, the sigma bonds, the hybridization and geometry tells us it will usually be a zigzag with wedges and dashes on the zigzag.
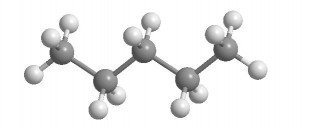
Notice that only the skeletal formula approximates the shape of the molecule? It's also the one that professional chemists use in journals, draw in class, and are easiest to discern. The type of molecular model set that is closest to this is shown below. The architecture and angles are obvious and this is the type of model we suggest you use in learning organic chemistry. The most important information a molecular model set gives is its conformation or shape. In general, the smaller, the better. It will look like the following:

The ball-and-stick model isn't realistic because it stylizes the overlap of bonding orbitals that make up bonds. The Ball-and-Stick Model is useful because of its clarity. Too much realism is counter-productive.
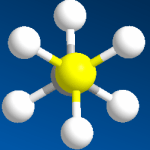
In contrast, the space-filling model is closer to the actual surface of molecules (which is more like a blob). The angles are hard to see and even the number of carbons isn't obvious. This model set has unnecessarily large and heavy atoms with flexible bonds that don’t attach firmly. The combined weight causes this type of model set to just fall apart in your hands. Save your money.
[caption id="attachment_3148" align="aligncenter" width="300"]
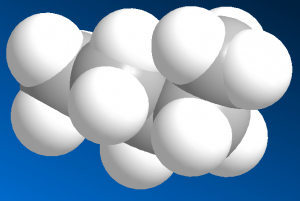
Space-Filling[/caption]
[caption id="attachment_3149" align="aligncenter" width="300"]

Realistic? Maybe. Useful? Not for this class.[/caption]
Some people prefer this type of model set. They aren't organic chemists. Space-filling model sets are often for display! Even biochemists who care about realism will model a protein or the shape of active site of an enzyme but computer simulations and X-ray crystallography do a much better job anyway. Big heavy models are usually just glued together and then stuck in some corner as a decoration.
[caption id="attachment_3659" align="aligncenter" width="310"]

Sheldon? Many things but no organic chemist.[/caption]
When To Use a Molecular Model Set
Professors often allow students to bring models to exams. Very few occasions require a model set during an exam. Unless you have severe problems imagining in 3-D (if you did, you probably wouldn't walk straight either) working with the model beforehand is enough. A model set gives you a good idea how to draw. Graders don't like hard-to-read drawings and it may influence your score. In general, it's better to just avoid models during exams, with two potential exceptions.
Use the model as you study and answer problems.
Ideally, you've already made extensive use of model your set before you step foot into an exam. Make a different molecule each having a different functional group) You'll be too busy drawing to have time to build models during an exam. Using models at exam time suggests you haven't worked enough problems. In contrast, bringing a few small, pre-made structures to the test can be useful if you feel like you need it for a particular type of question mentioned below. If it gives you a boost of confidence to have it nearby, well then bring it along.
At the start of the semester, long before any exam, build key structures and manipulate them for a couple one-hour sessions. Follow the instructions that come with the model as they usually have examples to make as part of knowing how to use them. Use the instructions, don't just build randomly. Besides boring you to tears, you'll get nowhere. Instead, make a goal to build about a dozen compounds. Make sure they each contain at least one different functional group. This will help you learn the functional groups and their names at the same time. Later, during the semester, you should pull out the model set again for topics related to conformational analysis (cycloalkanes) and stereochemistry (chiral centers). These are the types of things to build that are most relevant.
Advice For Using Your Molecular Model Set
1) Don’t skip hydrogen atoms. Always fill every valence position on every atom when building models. Be meticulous and the time you spend will benefit you. When you are doing organic chemistry problems on paper, you won’t need to build models again unless you wish to. Take it seriously and you will more easily associate 3-D geometry in 2-D. Don't assume this is somehow for children or "beneath" you. Indeed PhD candidates and senior pharmaceutical chemists rely on this when planning a synthesis even though computer modeling programs are available.
2) Don’t go through the instructions then just put the set away. The instructions will have you make a few molecules just to show you how. Do not stop there. Pick specific small molecules from your book that have unusual looking functional groups and make them (until it begins to feel natural). As final challenges, try norbornene, adamantane, and cubane. These are small but unique, even bizarre, in structure. If you didn’t know what they are, Wikipedia has the Kekulé structure, physical properties, and other information about common molecules, all those you will study in your organic course and lab. Enormous journal databases like Scifinder, STN, and Beilstein are routes to all the other known organic compounds (>10 million). The more you make the easier it becomes and gives your molecular model set value.
Besides giving you a realistic and scale model of the compound, the model helps you to begin imagining similar molecules in your mind in 3-D. Rotating around single bonds alters the shape or “conformation” of the molecule. This is important. The potential energy of the molecule changes with conformational changes and the data obtained is known collectively as conformational analysis. This is a fancy sounding term for the study of how geometry and energy are related to the course of a reaction within a molecule or between molecules.
A molecule can exist in different shapes and may have limitless possible conformations. Not all are stable. All of these, whether are exist readily (molecules rotate and move quickly and constantly we try to use the the averaged over time) or not are called conformational isomers (a different form or shape of atoms connected the same way).
For example, there are no conformational isomers of the molecule methane (CH4) since there are only four C-H bonds. Rotation of a C-H bond changes nothing about methane’s overall shape.
[caption id="attachment_3660" align="aligncenter" width="214"]
Bond Rotation = same conformer[/caption]
However, if you replace a hydrogen (-H) on one of the carbons with a (-CH3) group, you’ve got ethane (C2H6 or CH3CH3) and the ability to rotate around a C-C single bond.
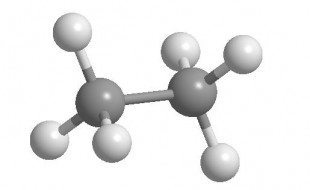
In reality, it’s so fast at room temperature, it’s like a blur (occurring approximately 1015/sec, or on the femtosecond scale.)
[caption id="attachment_3163" align="aligncenter" width="150"]
Staggered Ethane[/caption]
[caption id="attachment_3164" align="aligncenter" width="150"]
Eclipsed Ethane[/caption]
From this perspective, the molecule assumes a quality where the interactions between bonds and atoms are clearly visible even though one carbon is hidden or eclipsed. This type of perspective is known as a Newman Projection in honor of Professor Melvin S. Newman (1908-1993). It is important that you understand what it is, which atoms are in front and back (you'll be told), and how to reproduce it on paper for any molecule requested. Which do you suppose is the more stable conformation of ethane, staggered (left) or eclipsed (right)? As carbon-carbon bond rotation occurs, the potential energy of ethane changes with the degree angle. Bonds and atoms like to be far apart. This is the basis of conformational analysis...and in more complicated cases, this is precisely one of the things people have a hard time imagining in 3-D. You'll at least have observed it once with a molecular model set.
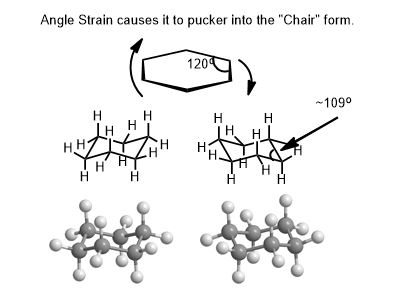
The Most Useful Thing to Make is a Cyclohexane Ring.
If you are allowed to build models during an exam, this along with any other groups. Different colors are recommended.
There is more background to cover before explaining the importance of this vital 6-membered ring to your understanding of molecular shape. It is covered extensively in conformation isomerism. Try building cyclohexane as an exercise. Don’t forget all the hydrogen atoms. How many should there be on each carbon?
Ask yourself: Is it easy to make all the carbon-carbon single bonds lie in the same plane? Is a 2-D hexagon shown likely? Only a model will give you tangible experience with chair cyclohexane. You will have to flip it to find out which is more stable.
The Other Useful Thing about A Molecular Model Set
Stereochemistry: You will be asked to identify whether any carbons in your molecule have four different groups attached to them. If they do, then that carbon is, by definition, chiral (KY-rul) or a stereocenter.
A chiral molecule (anything chiral) has a mirror image that is not the same itself (as the object in the mirror). It sounds odd, but think about your hands. Your hands are chiral. If you put our right hand up to a mirror, you see what appears to be your left hand in the mirror. But the image of a left hand is not your left hand, it is your right hand. Right?
You therefore cannot "superimpose" the back of your left hand over the back of your right hand and have the fingers be in the same order. As anyone who’s gotten dressed in the dark knows, gloves and shoes do not fit properly on the wrong hand or foot. Atoms with 4 different groups on them have this property and each is a pair of enantiomers. It is very difficult to tell them apart if you have just one of each type. Assigning a name to enantiomers that everyone can understand and do is necessary (R/S system). A model does help especially in certain situations.
[caption id="attachment_3168" align="aligncenter" width="300"]
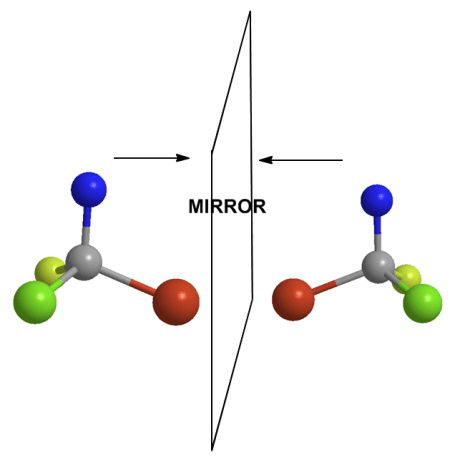
These molecules can't be superimposed so that all colors match atom to atom. Each is chiral.[/caption]
When you get to the chapter on stereochemistry and chirality, a model may be useful but certainly isn't necessary. If you like, you can make and bring a few small models before your exam and more easily determine whether the drawings (usually of cyclohexane (above) are identical, stereoisomers, or different conformations of the same thing: a very common question on exams and assignments.
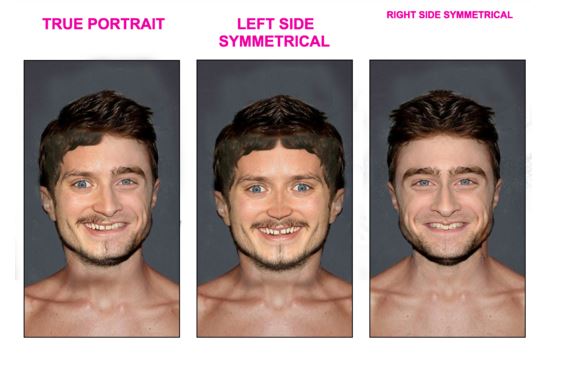
Oddly, our faces are not actually as they appear in the mirror. No face is perfectly symmetrical. Two halves of the same face are always different in some way. You can see this if you flip the right side of your face onto the left half...and do the same thing with the left side of your face...you'll see two very different looking people.
The result is that other people can see "the real you" but you never can (unless you set up two mirrors properly). Every time you look at your reflection in the mirror, the image in front of you is not the "you" that other people see. It is not how you appear to others. But that face is what you've grown used to seeing. The person who is your mirror image is therefore not "you". Basically you've only ever looked into the mirror and seen your enantiomer but not "you". If you saw what others see you would notice an unfamiliar face and do a double take. Chirality is a broad and abstract concept and this is one idea to think about for now.
Here is an obviously contrived example from the web of a hobbit's face. Anyway, you get the idea.
[caption id="attachment_3796" align="aligncenter" width="563"]
Source: Imgur[/caption]
Designing a Synthesis: Much later on, you will learn how to make organic compounds, step-by-step, one reaction after another to arrive at a desired target molecule. After all, there is a point to learning all these reactions. Targets are often of biological or industrial importance. Pharmaceutical compounds can be enormous requiring not just one but three to five molecular model sets to construct. Not building models of sections or the whole molecule can be fatal when deciding on a potential synthetic sequence of reactions. Computer programs fill the role of molecular model set as well. Baby steps first!
In 1953, James Watson and Francis Crick determined (from info they borrowed without asking) that the genetic material is a double helix. It brought them world renown and they shared a Nobel Prize. How?
They built a model! More importantly, the structure instantly suggested a very likely way to react or mechanism for DNA to duplicate. Already well-established rules for base pairing made this possible at the time. Structure and function are intimately related. It is a common theme in organic chemistry and biology. Is your molecular model set important? Yes, and if you want to gain as much insight as possible you will make use of your molecular model set early and often.
...ahem...then sell it for a profit.


Ready to conquer organic chemistry with confidence? Explore our services and resources now to start your journey towards success! Join our community of learners and unlock your full potential in organic chemistry. Let's embark on this exciting journey together. Get started today!
Read More