Constitutional Isomers
Constitutional (formerly Structural) Isomers
Isomers: n. chemical species that share a feature in common yet differ enough to be a discrete entity.
The largest class of isomers (there are three kinds) are constitutional or "structural" isomers. When two molecules share the same chemical (molecular) formula, yet have bonds that connect atoms in a different order, they are constitutional isomers.
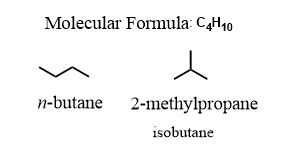
For example: Draw all the consititutional isomers of C4H10. This formula is in agreement with an important general formula for alkanes, CnH2n+2.
Just sitting here and thinking, I can draw two of them. I can shorten the chain by one carbon and place it as a branch (a substituent) on the only carbon where it will make a difference.
I can't however make it into a ring or put a double bond in it. Consider what will change, if I do either of those things.
If I draw cyclobutane (a square) for example, i am not drawing an isomer of butane. I am drawing unrelated compound and not an isomer, as isomers are easily defined: Compounds that have the same chemical formula. To make a cyclic compound, an H atom must be removed from the ends and the ends bonded together. This is a loss of two hydrogen atoms or an "unsaturation". Alkanes are saturated with hydrogens until you put π-bonds in them.
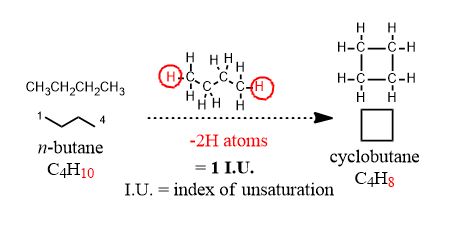
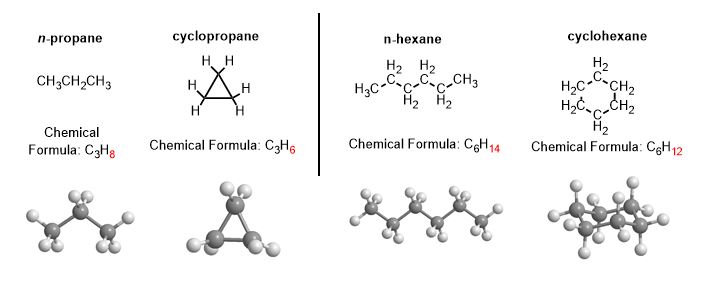
Butane and cyclobutane are technically unrelated! They don't have the same molecular formula and so have no isomeric relationship. Ditto for other chains and rings.

Ready to conquer organic chemistry with confidence? Explore our services and resources now to start your journey towards success! Join our community of learners and unlock your full potential in organic chemistry. Let's embark on this exciting journey together. Get started today!
Read More