Cycloaddition Reactions
Cycloaddition Reactions
Cycloaddition Reactions are among the most versatile and useful pericyclic reactions in Organic chemistry.
The most common cycloaddition is the [4+2] or Diels-Alder reactions. Four pi electrons meet with two pi electrons and having a symmetry match is allowed, so to speak, to occur. Two separate compounds one with two double bonds and with one alkene or alkyne and they come together to form a cyclic product or "cycloadduct" (adduct = addition product").
Just like nucleophiles are HOMO's and electrophiles are LUMO's, the diene must be a HOMO and the dienophile must be a LUMO. (This is backwards sometimes)
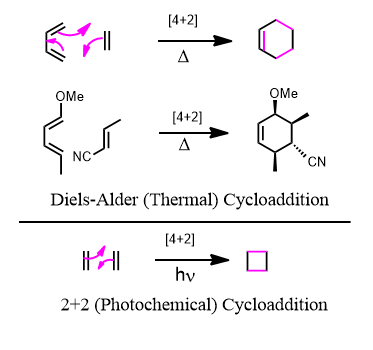
Starting with a diene (four pi electrons) and a dienophile (2 pi electrons) the product in the forward direction is a cyclohexene. This means that since the reverse reaction is also spontaneous under the right conditions, any compound containing a cyclohexene ring is subject to cycloreversion and the microscopic reverse process back into a diene and a dienophile.
Just because it says heat, doesn't mean it has to be scorched and beaten. Room temperature is often sufficient to trigger the reaction. Since you end up with two sigmas and a pi in exchange for three pi bonds, the cycloadduct is more stable since sigma bonds are stronger than pi bonds.
One can draw the mechanistic arrows in either of two ways (both correct):

Ready to conquer organic chemistry with confidence? Explore our services and resources now to start your journey towards success! Join our community of learners and unlock your full potential in organic chemistry. Let's embark on this exciting journey together. Get started today!
Read More