A Word about Organic Oxidation-Reduction Reactions :
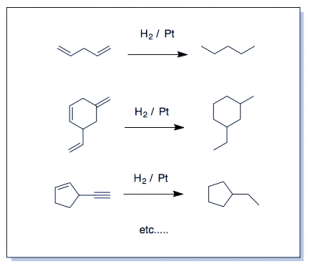
Realize which are the "easy" reactions and those for which you MUST draw the mechanism. The easy reactions are the Oxidation-Reduction Reactions. (You memorize or "flashcard" these.) Learn to recognize these and you're life will simplify enormously. The several oxidation-reduction reactions which are the ones you actually "memorize" by rote, will clutter your thinking about substitution and elimination unless you get this. There is no point in drawing an arrow-pushing mechanism for these reactions—not at the undergraduate level. Many redox reactions are not still not that well understood even at the highest levels. Learn what such reactions do and simply apply it to everything with the same functional group...it is hardly a coincidence that oxidizing agents contain a lot of oxygens and reducing agents with hydrogen atoms delivered in various ways. Remember this on exams. Oxidizing agents have a lot (or only) oxygen atoms and reducing agents have several (or only) hydrogen atoms. See Organic Oxidation-Reduction reactions if you don't understand what it means to oxidize or reduce a molecule in organic chemistry. Hydrogen is added and this is the definition of reduction on carbon (it gains electrons relative to the loss of oxygen (electronegative) atoms). Rarely are you asked to draw the mechanisms for (hydrogenation (H2/Pd), step-wise reduction(LiAlH4, NaBH4, ozonolysis (1.O3; 2. oxidative/reductive quench), epoxidation (RCO3H; mCPBA-see below), or, for example, syn-dyhydroxylation which adds two hydroxyl groups across an alkene (using cold KMnO4, KOH or 1) OsO4 2) NaHSO3). These are reactions you simply just do at the functional group in question, not something you figure out. H2 and palladium (Pd) catalyst does a similar thing as dihydroxylation except that catalytic hydrogenation (H2/Pd or Pt) is a reduction—addition of -H atoms are added to a single face of the double bond. (See oxidation-reduction reactions). If hydrogen is added to the molecule intentionally, it is a reduction (catalytic hydrogenation). It appears as though π-bonds just vanish and for that reason is possibly everyone's favorite reaction.)
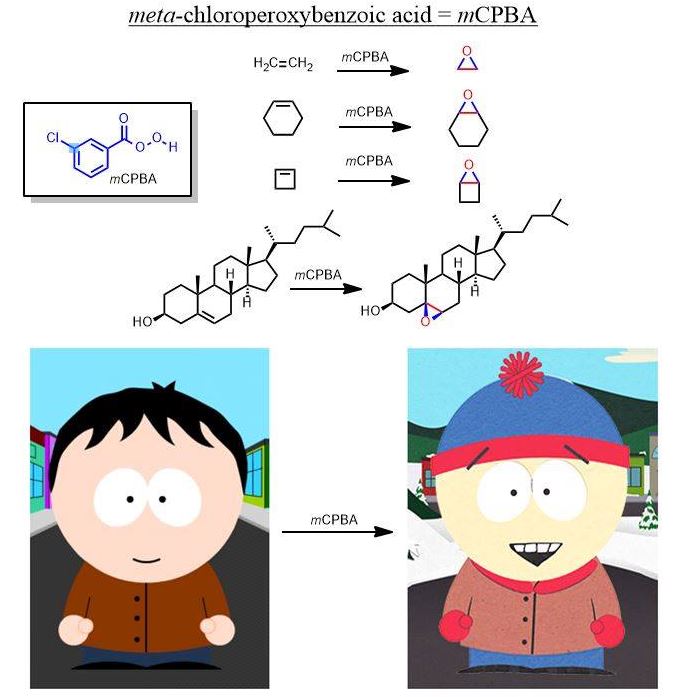
As another example, this is what mCPBA does to alkenes in an oxidation:
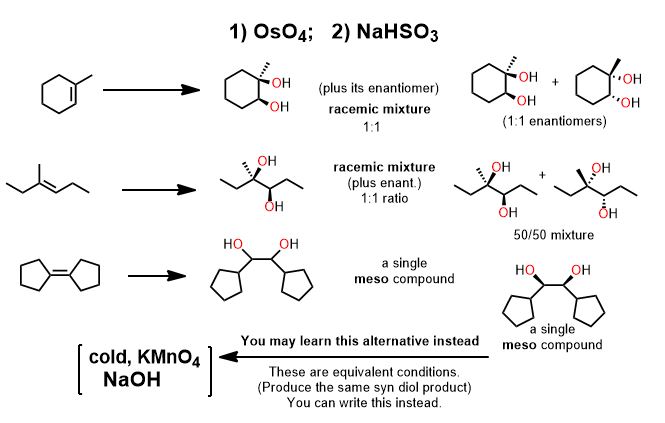
The mode of addition for many redox redox reactions is syn. The product has both groups added such that they end up cis to each other because the atoms come from one face, the one reductant or oxidant molecule. To add them otherwise (anti) is geometrically unfeasible.

Ready to conquer organic chemistry with confidence? Explore our services and resources now to start your journey towards success! Join our community of learners and unlock your full potential in organic chemistry. Let's embark on this exciting journey together. Get started today!
Read More