Periodic Table
What you need to remember about the Periodic Table
The periodic table is convenient because it’s built around trends. These trends were essential in general chemistry and they are essential now in Organic Chemistry. Not all of them are important to remember.
For the most part, you know that the more more electronegative you are, the less metallic you are. That’s why the elements that make up life like carbon, nitrogen, oxygen, hydrogen, are all non-metals. Hydrogen may in be Group IA (the alkali metals) but it does not behave anything like the rest of its family.
The trends in the the periodic table that are most important are:
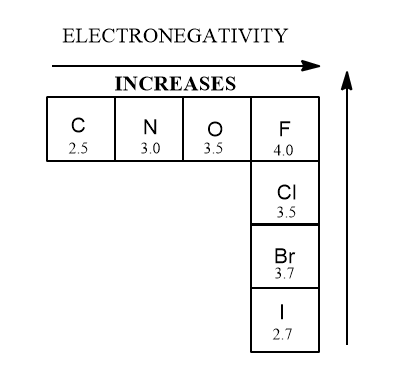
- electronegativity
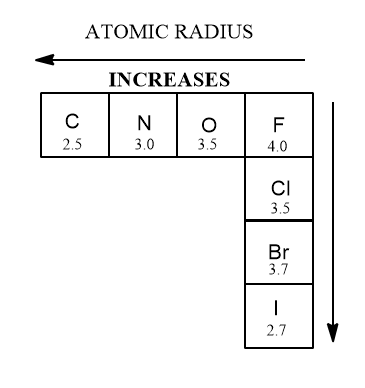
- atomic radius (which is related to polarizability)
The reason this is so is because acidity of atoms, a critically important topic, is based on these trends in these atoms. Most people remember that fluorine is the most electronegative element because it is and because of this trend.
The other trend in the periodic table that’s important for acidity in Organic chemistry: atomic radius. The larger an atom is, the more acidic it is. Also the “squishier” it is. In general, the squishier the nucleophile, the better it is. I- (iodide) is a superb nucleophile but that comes later.
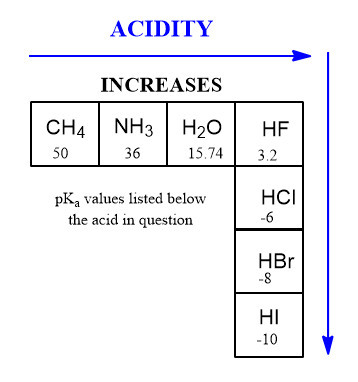
As far as Acidity and the Periodic Table is concerned:
The trends that matter in the Periodic Table. Review the article on pKa and acidity. There’s two of them. (That’s it).
The pKa scale is simply the mixing of these two trends. As you go from left to right in the periodic table you get more acidic, as you go from top to bottom you get more acidic. This means that the more electronegative you are and the larger you are, the better you are at donating protons (to bases).
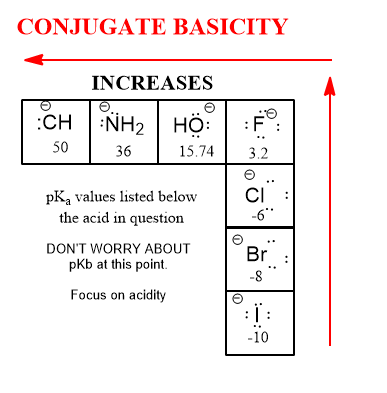
Acidity is related intimately with corresponding basicity by an inverse relationship that makes an acid that reacts into its conjugate base. The base of course, has a strength proportionally weaker as a base due to the willingness of the donor acid. The stronger the acid, the weaker is the conjugate base. The weaker the acid, the stronger the conjugate base. This only makes sense:
An acid’s job is to give up or donate a hydrogen atom. When it does, it rarely has more to donate lest it become way too unstable (it has a humongous barrier to activation and doesn’t normally happen). If it really wants to give up an H+, do you suppose it wants it back? Of course not. This is the simple logic of an acid’s strength in relation to the strength of its conjugate base (itself minus an H+).
You’re thinking, there must be an important trend that goes up and to the left, perhaps. There must be and it is the strength of the conjugate bases of these acids.
The trends that matter in the Periodic Table. Review the article on pKa and acidity. There’s two of them. (That’s it).