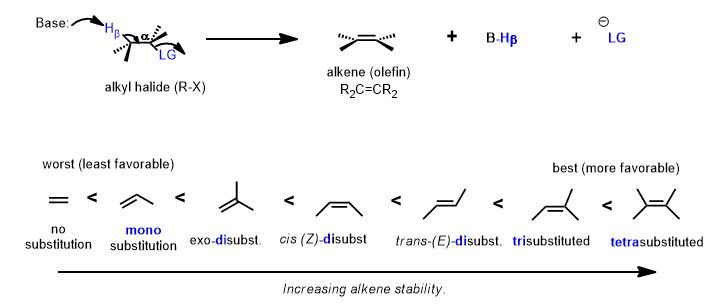
Zaitzev's Rule
Alexandr Saytseff or Alexander Zaitzev (Romanized) was a student of renowned chemist, Vladimir Markovnikov. In the middle of the 19th century, (ca. 1865), an observation led to an eponymous regiochemical term. The rule, when applied, determines, in most cases, which alkene will be predictably major in an elimination reaction (E1, and if geometrically favorable, the E2) and the major regiochemical product is known in his honor as the "Zaitzev product". The higher regiochemically-yielding product. The memory device or mnemonic device, as it's now known, is: "the poor get poorer". The beta-carbon bearing fewer hydrogens will lose the hydrogen in the elimination of H-X.
Recall that Markovnikov's rule (the opposite process of breaking an alkene double bond to give a carbocation) states that the rich become richer. Because, at the time, we were making carbocations. The more substituted with carbon it is, the more stable it is. The more substituted the carbocation, the faster it forms and the faster the reaction goes (it's rate-limiting).
The carbon with more hydrogens gets more hydrogens or "the rich get richer" (he was talking about H-X but the mechanism is for whatever the electrophile...like boron (B) in hydroboration/oxidation (BH3). What he said was that in the electrophilic addition of H-X to a pi bond, the electrophile goes to the carbon bearing more hydrogens (the less substituted hydrogen." This leaves the carbocation to be formed at the more substituted position. Recall that for carbocations, the tertiary > secondary > primary >> methyl cation. This is due to the phenomenon of hyperconjugation.
We are doing the reverse reaction here and forming a double bond to form an alkyl halide. However, the reason for the selectivity is also hyperconjugation. The rule of Zaitzev also has a memory device dealing with the number of hydrogens (at the beta-carbon) and the quick and dirty mnemonic is that "the poor get poorer". The carbon with fewer hydrogens must have more substituents. Therefore, when the pi bond forms, the more stable (i.e. more substituted) olefin (above) will be formed preferentially, not this one below. This is only part of the story. That is Zaitzev's Rule.
Look at the alternative β-hydrogen (it's on methyl which possess three, rather than one, hydrogen atom—the one that is poorer in hydrogen atoms is the one that must have more carbons attached to it and therefore makes a more substituted double bond.
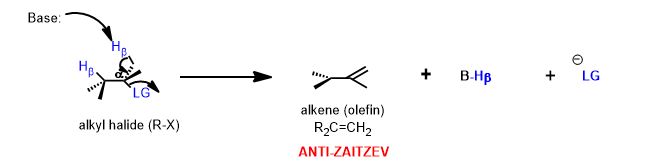
Since the methyl carbon that is also beta to the leaving group can be removed, you have get the exo-disubstituted alkene as well. However, it is less stable (has larger barrier to its formation) and is minor in terms of the product ratio relative to the major product above. This rule is operative whether the reaction is E2 or E1. Only a different reaction mechanism can yield the anti-Zaitzev product, i.e. the Hoffman elimination or the Cope Elimination.
What else will trigger the E2 reaction?
KOt-Bu, or potassium tert-butoxide is a sterically bulky base, as such, it is a terrible (incompetent) nucleophile.

Ready to conquer organic chemistry with confidence? Explore our services and resources now to start your journey towards success! Join our community of learners and unlock your full potential in organic chemistry. Let's embark on this exciting journey together. Get started today!
Read More