SN2/SN1 E2/E1: The Simplest Method
Identifying Classes of Substitution and Elimination
You are in Organic 1 and are learning about alkyl halides. These substrates have sp3 carbons bearing good leaving groups. Your job is to get these off of the molecule in the correct way. You can therefore 1) replace the leaving group and substitute it for some other atom or group (SN) or 2) Eliminate it with a neighboring (or "beta") hydrogen atom to form an alkene.
Where's the Confusion?
1) Regardless of what a question asks for, you should be doing or attempting to do both of these reaction classes on all alkyl halides presented to you (although it seems there are hundreds of reactions there are only 5 classes of reaction in Organic 1! ...tons of alphabet soup, but only 5 major reaction types). If you've had calculus think how simple it would be if there were only the 5 rules involved (e.g. the chain rule, product rule, Newton's method etc.). Just like in calculus or algebra, all else are variables: the starting material, the reagent, and the product. In this sense, I see, as you will too, that organic chemistry is chemical calculus. The difference is the processes are called mechanistic hypotheses (mechanisms) not mathematical rules.
2) The greatest confusion arises in determining the kinetics of the substitution or elimination reaction you are doing as each has two different pathways: uni- and bimolecular (i.e. is it E1 or E2?)
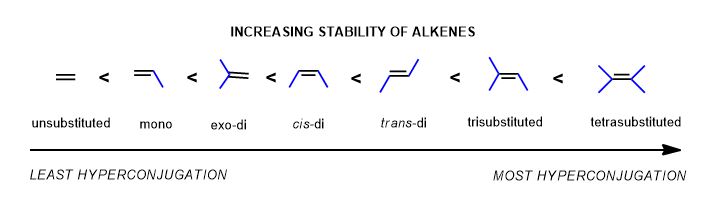
For the elimination reaction, the product will depend on its stability as an alkene—how substituted it is. The general guideline for determining the major product is Zaitzev's Rule.
It's simply a statement and memory device about the placement (regiochemistry) of the alkene in the major product. It will be the more thermodynamically stable (more highly substituted) alkene. This is the basis of the mnemonic device that bears Alexandr (Sasha) Zaitzev's name "the poor get poorer". Where there are fewer hydrogen atoms, there must be fewer carbons attached. So when it is abstracted by a base, there will be more neighboring carbons in the resulting alkene. This is common sense.
Thus the major products are based on the following trend: the more substituted (the more blue bonds there are to) the carbons of the double bond, the greater is the electron delocalization (hyperconjugation) and therefore, the more stable and readily-formed is the alkene. (There are steric considerations, but let's ignore those for now.)
However, as you are fully aware, more than just elimination is possible. You've reached the four limiting substitution and elimination reactions (SN2/SN1 and E2/E1). There is nothing magical or mysterious about these after all you've been through already. You've performed them in different forms already if you've done alkene chemistry and even if you don't realize it.
Let's take a short trip down memory lane at what you've endured: To be at this point means you've already covered the majority of the concepts in the course besides possibly NMR spectroscopy. Taking a look back... You've covered bonding and hybridization, nomenclature, acids and bases, mechanisms like alkene Addition, different types of isomerism, stereochemistry, resonance and other conceptually difficult topics.
There are few new reactions remaining. The focus for the remainder of Organic I, using elementary steps you've already performed, rests on single-bonded functional groups, alcohols, ethers, epoxides, sulfur-derived esters of alcohols (so-called pseudo-halides: tosylates (-OTs), mesylates (-OMs), triflates (-OTf), thiols, and possibly amines. The rest (substitution and elimination) should be a cake walk. But it isn't. You feel a looming shroud of uncertainty about the "order" or "molecularity" of the SN and E reactions you happen get and which to do.
You may not be sure which one to apply. If you aren't clear whether the reaction is unimolecular (first-order) or bimolecular (second-order) (Was it SN1 or SN2?) or when to substitute and when to eliminate; then you will not be able to know the product or its stereo- or regiochemistry. That's a lot of partial or total credit riding on a coin toss.
The substitution and elimination reactions are no more complicated individually than the addition reactions to alkenes that create the alkyl halides you're now dealing with. Indeed, the unimolecular substitution (Sn1) and elimination (E1) involve the same carbocation intermediate and in fact, the E1 is essentially Electrophilic Addition to alkenes except in reverse.
The large difficulty, on the other hand, is determining what the molecularity of the reaction is. (Is it a first-order (unimolecular) or second-order (bimolecular) reaction that you're doing?).
It is the differentiation of these 4 reactions that cause students to literally bang their head against the wall. Yes, I've witnessed it and done it. But there's a reason for that: the way it is invariably presented. From a teaching (and learning) standpoint, it is here that one of the largest blunders is made by textbook authors (all of them; and for decades). They ought to grouped as SN2/E2 and SN1/E1. Clearer about that? Below, and again in part 2 of this article you'll see what is meant by this.
Let's try clear that up, but first: (skip over the following digression if you've read about Oxidation-Reduction reactions already).
With the exception of radicals, organic chemistry is acid-base chemistry or the interaction of oppositely charged species. Charge (whether + or -) is, by nature, unstable. Ions are therefore, KINETICALLY REACTIVE and are attracted to or desire to react with something else. CHARGE is a high energy BURDEN. If you are making choices that reduce the burden, you are thinking correctly.
In the first and rate-determining-step, on the far left below, the halide (-X = Br = Cl = I) leaving groups just goes away with one arrow in the curved arrow formalism. Hydrogen bonding to protic solvent assists to remove Br- and stabilizes the ion (anion) formed.
Consider the reaction of (S)-3-bromohexane with methanol. The mechanism is unimolecular. How can one tell?
I knew it must be, as you should and are about to learn, how to make this determination. 1) the presence of methanol 2) the ability to make a reasonably stable carbocation is the reason...Is methanol a strong base or good nucleophile?
It's weak. Neutral. Then you can only do unimolecular (SN1 or E1) reactions. Get it? The reagent (in undergraduate organic) always gives you the best clue as to the answer. Of course, you must make sure to draw the arrows that get you where you are thinking. You mustn't make terrible carbocations, and also realize carbocations spontaneously rearrange to have more hyperconjugation (stability).
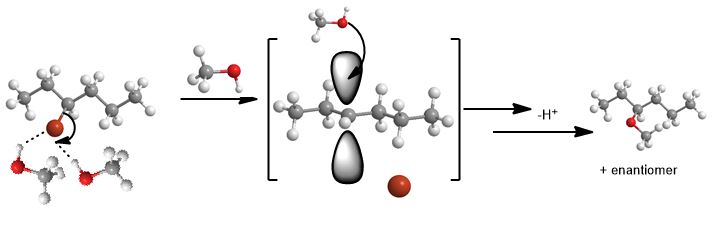
[caption id="attachment_3983" align="aligncenter" width="687"]
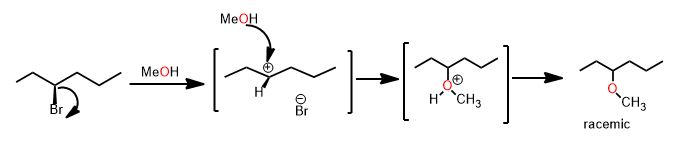
The SN1 (Unimolecular Substitution)[/caption]
There is solvolytic attraction of the bromide leaving group to a protic solvent until the point of complete ionization and hydrogen-bonding to water or the hydroxyl group on an alcohol. Solvent makes them stabilize by organizing with the surrounding solvent. That's the whole thing, both steps. Nevertheless you find there's something more complicated about these reactions—1) there's more of them 2) each has special rules or exceptions and 3) somehow it always seems more complicated than it is. While its a good guess, the nature of the solvent alone is not what determines the reaction mechanism. It's even simpler than whether the leaving group can hydrogen bond to solvent or not. It's because alcohols are poor nucleophiles. You know this because it is neutral, like water, which is also a poor nucleophile.
With the exception of multi-step synthesis, you're really on the home stretch to the finish line. Often there are professors who will tell you beforehand what the mechanism is in the question. For example: "Give the SN2 product for the following" or "Draw all the E1 products for the dehydration of alcohol Y and circle the major one". If you are going to be taking the ACS examination, I'll tell you in advance its multiple-choice and tells you what mechanism is involved. So, the ACS Exam Organic Chemistry Exam also does this but an exam and course content are orthogonal having competing interests. Regardless, if you're facing the ACS exam as your final, be relieved because it is usually easier than your regular exams and not just because it's multiple choice.
The majority of professors should not (and thankfully don't) examine students by revealing the mechanism in the question. That may sound condescending but is not meant to be. You aren't told what reaction to do. How do you know which to do? You will need to learn to fly on your own after this because organic synthesis (which is coming) makes you the question author.
Anyone should and can learn to know and predict the difference without being told a ton of other things that matter not one iota to an undergraduate organic student. Soon what reactions and mechanisms need to be done will be your job, to come up with an organic synthesis (make a larger compound from smaller ones) and the reactions and the mechanisms are of your choosing so you must be able differentiate between them. If you can't, what reagents will you write in?
Take just the information above and see if you can do the following (solutions below):
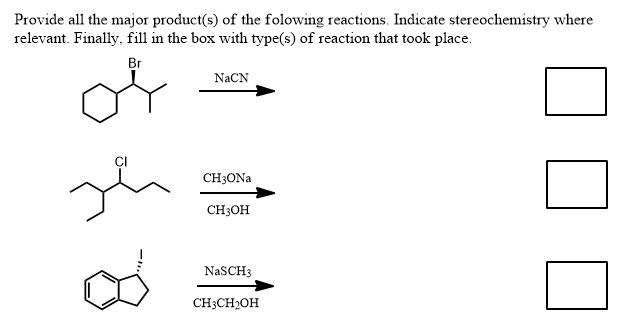
So, what are we looking for exactly to solve these kinds of problems?
For Substitution and Elimination You're Asked For One or All of These:
1) Given the structures of A and B, provide C, or some variant of that..Given compounds B and C, provide the substrate A, etc.
2) Identify the mechanism based on the information provided.
3) Provide all the reagent(s) in the right order if it is a multi-step sequence when only A and C are provided.
4) Given a molecular formula (CwHxOyNz...) for A, and a series of hints about about the conditions (B) that provides product C, give the structure of A.
...etc.
What Matters and What Doesn't?
On our list of unspoken rules this one concerning substitution and elimination (SN2/SN1; E2/E1) occupies a large space.
Notice you can't answer any of these until you understand what determines whether the reaction is unimolecular or bimolecular?
This is the form of the reaction you do: A + B goes to C. For these chapters on (direct) substitutions and eliminations, The substrate "A" is R-X or an alkyl halide. B is your reagent (base (elimination) or nucleophile (substitution)), and whatever the nature of B is, it will reveal what kind of reaction you are restricted to. Note that some professors may occasionally switch the positions of "A" and "B". This is a curious and common source of confusion to many who still aren't able to identify what's the electrophile and what is the nucleophile.
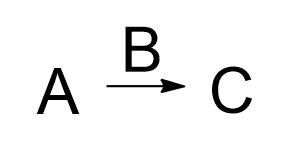
There are exceptions, of course:
1) Multi-step synthesis. Take the above equation and, as simply as it can be put, keep repeating it using the right reactions in the proper sequence, until you reach the target molecule. That's the above formula repeated over as few numbers of steps possible.
It might appear to the beginner that that is the idea, but in reality, organic chemists do not do things in this forward direction. 1) They do it in backwards or "retrosynthetic" fashion. Meaning they start with the ultimate target and work step-by-step backwards. 2) Organic chemists who want an efficient yield do not follow these steps in a linear fashion if possible.
Everything in between A and An (ω or TGT or just 'the target') is an unknown. Don't get scared. The people who say there are hundreds of reactions to memorize in this subject simply didn't know what they are doing when they took it and still don't know what they are talking about afterward. Sure there is a lot of alphabet soup in reagents but those are things that do one thing to usually just one type of functional group.
2) Given A, B, and C, provide a reasonable mechanism, (D).
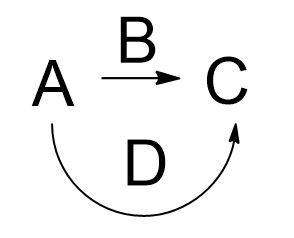
If you don't practice and draw reaction mechanisms (D) all the time while studying Organic Chemistry
you will not learn much of anything.
Drawing all of D is your job. Get that clear and you will solve problems rather than guess.
You've heard this before. Some supposed experts actually warn about trying to avoid memorizing the material as that is not a good idea in this subject. The reality is, you don't have a choice, memorization isn't an option, even if you try unless your prof is a total pushover...(I've yet to meet one). There are practical things and procedures, meaning they must be done to solve any of several questions that can be asked.
Think of Organic chemistry as a performance ability or skill. One cannot memorize a skill. Try it on a bicycle, which has only about two steps. Sit and pedal. Procedures of dozens of steps cannot be memorized. Read a hundred books about playing piano or how to swim, watch virtuosos, listen all day long to concertos, hang with Michael Phelps but with just fact-based familiarity, at the end of the day, it would be a miracle if you could play "chopsticks" or manage not to drown. One will just make semi-educated guesses about virtually everything. At the beginning, it's not so bad, because certain topics like nomenclature, isomerism, and spectroscopy are stand-alone, and not as cumulative and remember that's the intro to the intro of the subject. If you aren't familiar with mechanism, it may hurt or destroy your semester (and the following one).
What Parameters Determine if its SN2 or SN1? E2 or E1?
-
Characteristics of the Solvent - Protic or Aprotic
-
Leaving Group Ability
-
Good Nucleophile or Poor Nucleophile
-
Strong Base or Weak Base
-
Amount of Substitution at the α-carbon: (primary, secondary, tertiary, quaternary: 1o,2o,3o,4o)
-
kinetics and Rate Laws: Bimolecular (rate = k[R-X][Nu or Base]) or Unimolecular (rate = k[R-X])
-
π-Systems Neighboring the α-carbon
Not everything here will get you certainty. Which one(s) will?
- Solvent. The solvent is always polar, whether protic or aprotic. Like dissolves like. They should be somewhat polar because what reacts inside is polar. There are often ionic species involved. Unimolecular reactions occur in protic solvents but so do many bimolecular reactions. Solvent make no difference to you except some are protic (favors multi-step mechanisms; unimolecular and others aprotic (favors bimolecular reactions). The meaning of protic vs aprotic involves mainly hydrogen bonding. Though it alone is not how you determine the whether it is a unimolecular or bimolecular mechanism.
- Substitution at the α-carbon - is very important. The number of carbons attached to the carbon bearing the leaving group (halide anion) is what makes it 1o, 2o, 3o, or 4o. Tertiary is the most straightforward of these. You can't do SN Concentrations and kinetics...you're not given that as that alone would give away the answer. Neighboring groups of p-orbitals will give you a stable carbocation intermediate or a stable transition state...either one will increase the rate, but that won't change the mechanism.
Ask Yourself a Question.
For the remainder of the reactions you do...ask yourself this question about the reagent nucleophile or base: Is is strong or weak?
If it's strong, it comes as a salt (an ionic compound). That's it. If it's weak it comes as an alcohol or water. That's all there is to it. Consider this for a while. It should make sense...of some kind.
It should be perfectly clear in following post.
Solutions to the problems above:
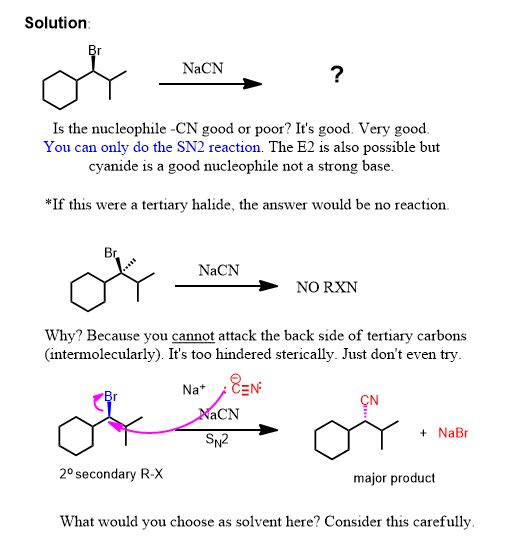
Second Problem:
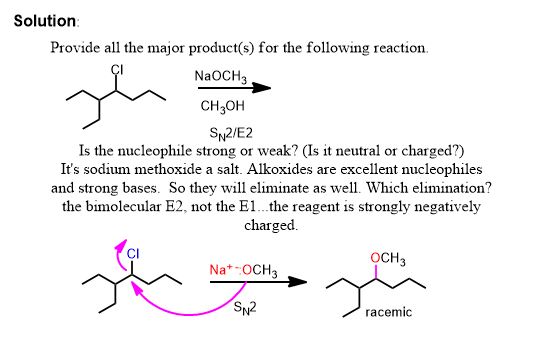
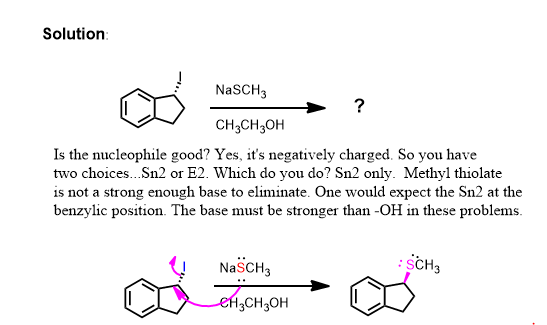
This problem isn't finished however. Yes there is a chiral center but it's configuration is not displayed so you do not have to care. It still remains chiral at that carbon but since it started racemic (straight bond) it must end up racemic. What haven't we done? The bimolecular elimination (E2) is in competition with the substitution because this is a secondary alkyl halide in protic solvent. Regardless, the reaction is SN2 even if the solvent is protic here.
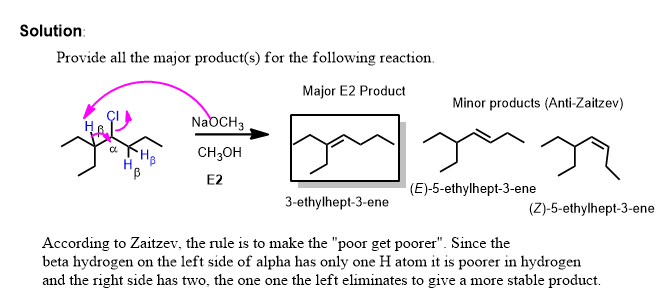
Thus the second problem has two equally reasonable answers and you must provide both unless told otherwise.

Ready to conquer organic chemistry with confidence? Explore our services and resources now to start your journey towards success! Join our community of learners and unlock your full potential in organic chemistry. Let's embark on this exciting journey together. Get started today!
Read More